Reviewing clinical considerations and guideline recommendations of C1 inhibitor prophylaxis for hereditary angioedema - Anderson - 2022 - Clinical and Translational Allergy - Wiley Online Library

These highlights do not include all the information needed to use TAKHZYRO® safely and effectively. See full prescribing information for TAKHZYRO®. TAKHZYRO® (lanadelumab-flyo) injection, for subcutaneous useInitial U.S. Approval: 2018

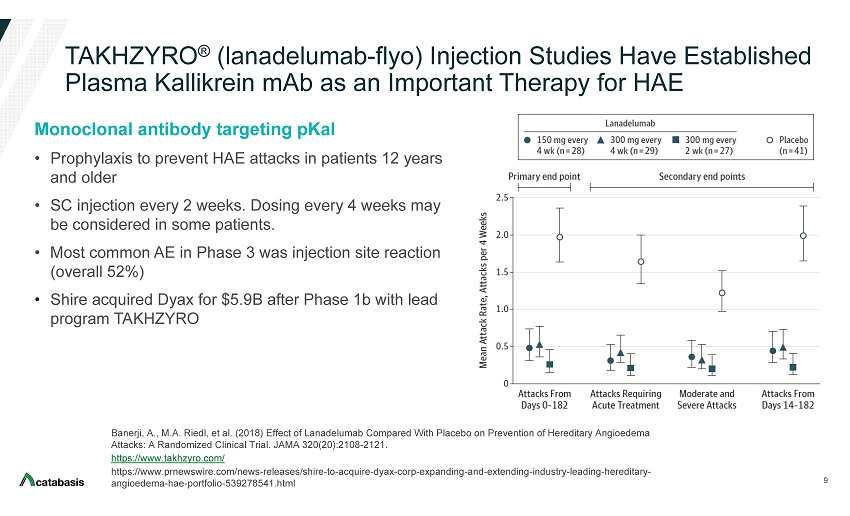
An open‐label study to evaluate the long‐term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension - Riedl - 2017 - Clinical and

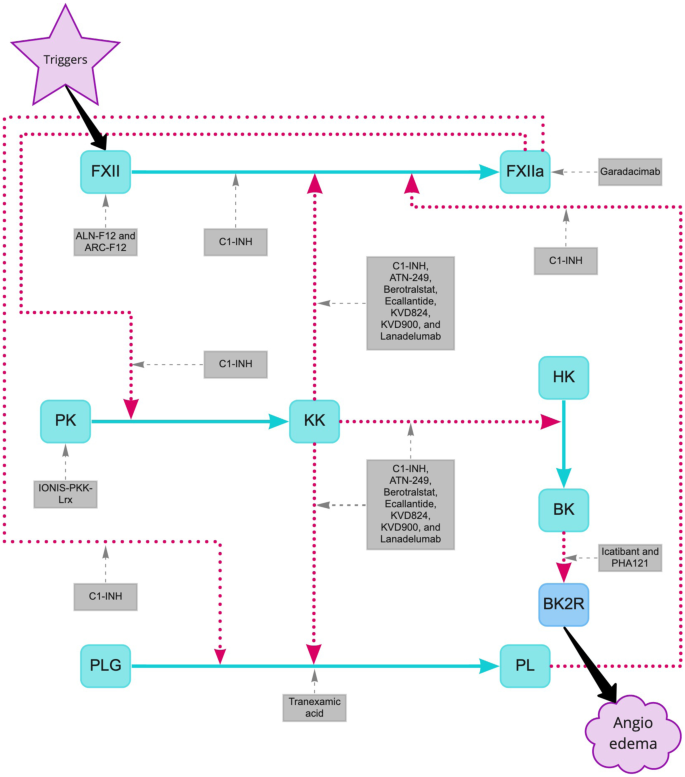
Frontiers | Comparing Pathways of Bradykinin Formation in Whole Blood From Healthy Volunteers and Patients With Hereditary Angioedema Due to C1 Inhibitor Deficiency | Immunology
Mechanism of action has an impact on antibody dose escalations. The... | Download Scientific Diagram

Recombinant replacement therapy for hereditary angioedema due to C1 inhibitor deficiency | Immunotherapy

Site of action of current and under research therapies for HAE treatment. | Download Scientific Diagram

Pasteurized and nanofiltered, plasma-derived C1 esterase inhibitor concentrate for the treatment of hereditary angioedema | Immunotherapy

US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema - The Journal of Allergy and Clinical Immunology: In Practice

Interim Phase 4 Data Support TAKHZYRO® (lanadelumab) as an Effective Treatment to Reduce Attacks in Hereditary Angioedema Patients | Business Wire